Börsipäev 14. september
Kommentaari jätmiseks loo konto või logi sisse
-
Rev Shark:
Breadth Number Highlights the Weakness
9/14/2005 9:10 AM EDT"Adversity reveals genius, prosperity conceals it."
-- Horace
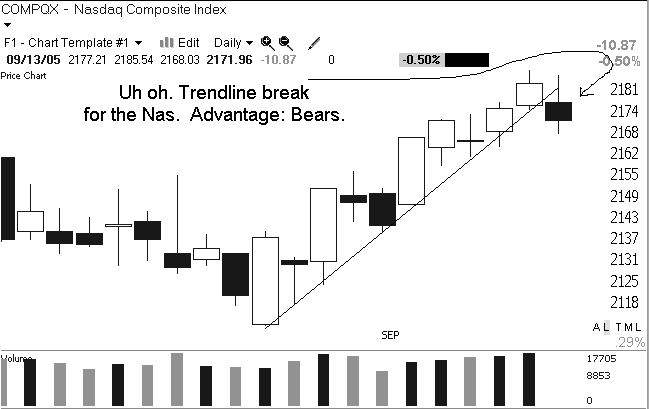
Yesterday the market confronted its first significant adversity since Hurricane Katrina hit. Until yesterday we had blithely moved steadily higher with little concern about oil prices and its economic ramifications, or the other concerns that have been in the headlines. The bulls have been telling us that if the market can act so well when there are so many negatives out there then we must be in good shape. The bears tell us that it is only a matter of time before the negatives catch up with us.
The issue we confront this morning is whether the poor action yesterday is the beginning of the end of this recent run or just a healthy pause to digest gains before we move higher. On the bullish side of the ledger we still have not suffered any major technical damage. Yesterday qualified as a "distribution" day because we pulled back on increased volume, but the market can handle a few of those before any real damage is done.
The major concern yesterday was the poor breadth. We had more than 2 decliners for every gainer, which tells us that there was some solid underlying selling pressure. Breadth is often a good indicator of the level of speculative action in the market. When more aggressive market participants step aside, the first to suffer are secondary stocks and smaller-caps. Those stocks aren't a big influence on the major indices so the only way to see them is to investigate breadth.
We are at a point now where you must be ready to act if you want to be positioned well. The biggest mistake investors make is failing to act quickly when storm clouds appear on the horizon. It is extremely important that we protect and preserve recent gains. Nothing is more debilitating to an investor than seeing hard-won profits disappear as the market rolls over and we sit idly by.
Market turns are what separate the good investors from the average ones; it is the folks who take action when the character of the market changes who establish a major advantage. Most investors do just fine making money when the market is acting well, but it is when it starts to struggle and roll over when our true investing abilities are tested.
Stay vigilant and be ready to act. Yesterday was a warning sign that we may be in for some bumpy action. If you want to produce market-beating returns this is the time when you have to make the most effort.
We have a slightly negative start this morning. Oil and gold are up, overseas markets mixed, and retail sales for August came in below consensus estimates.
Gary B. Smith:
-
Gapping Up
DISK +43% (receives unsolicited proposal from LGF), VISG +15% (partners with Motorola), CTIB +12% (extends yesterday's 186% move on a new product launch), WTRS +11% (extends yesterday's 13% move), CECE +6.5% (receives significant new orders), ENCY +4.3% (clinical data), KR +3.9% (multiple upgrades: Morgan Stanley, CIBC, KeyBanc), SCSS +3.6% (guides for Q3), ANTP +3% (extends yesterday's 17% move), BMET +2.7% (FDA issued approval letter), IDNX +2.6% (awarded statewide contract), LEH +1.8% (reports strong AugQ).... Under $3: NTWK +26% (reports JunQ), VRSO +32% (introduces Skype filtering technology), NWAC +7.6% (may file for bankruptcy as early as today).
Gapping Down
BIDU -24% (started with an Underperform at Piper and at Goldman; down in sympathy: SOHU -4.1%, NTES -2.4%, IIJI -2.4%, GOOG -0.8%), DPTR -6.6% (reports Q4), CEC -5.8% (guides below consensus for Q3; BB&T downgrade), LPMA -4.5%, PGNX -3.5%, GNW -2.5% (GE to sell 60 mln shares)... Under $3: APAC -24%, GNTA -14%, PRTL -8.4% (profit taking after 34% move yesterday). -
DNDN Dendreon to submit application for FDA approval of Provenge for treatment of advanced prostate cancer DNDN Dendreon to submit application for FDA approval of Provenge for treatment of advanced prostate cancer (6.45 +0.22)
Co announces plans to submit a Biologics License Application (BLA) to the FDA to market Provenge, the co's investigational immunotherapy for the treatment of advanced prostate cancer. This decision follows a recent pre-BLA meeting in which the co reviewed safety and efficacy data with the FDA from its two completed Phase 3 clinical trials of Provenge in patients with advanced prostate cancer. The outcome of these discussions determined that the survival benefit observed in the D9901 study in conjunction with the supportive data obtained from study D9902A and the absence of significant toxicity in both studies is sufficient to serve as the clinical basis of a BLA submission for Provenge. Co will host a conference call today at 4:00 p.m. EST. -
Hea uudis DNDN poolt. Siiski usun, et esimene spike müüakse ära nagu ikka.
sB -
Shorte palju sees.
-
DNDN +14% :):)